VOLUME I: DEFINING THE RIGHT ANTIBODY COMPOSITION
Therapeutic Antibodies - From Past to Future
Stefan Dübel
PART 1: SELECTING AND SHAPING THE ANTIBODY MOLECULE
Selection Strategies I: Monoclonal Antibodies
Gerhard Moldenhauer
Selection Strategies II: Antibody Phage Display
Michael Hust, Andre Frenzel, Florian Tomszak, Jonas Köller
Transgenic Animals Derived by DNA Microinjection
Marianne Brüggemann, Michael J. Osborn, Biao Moa, Suzanne Avis, Ignacio Anegon, Roland Bülow
Molecular Engineering I: Humanisation Strategies
Jose Saldanha
Antibody Affinity
Andre Frenzel, Lorin Roskos, Scott Klakamp, Meina Liang, Rosalin Arends, Larry Green
Molecular Engineering III: Fc
Thomas Valerius, Matthias Peipp, Stefanie Derer, Stefan Lohse, Christian Kellner
Glycosylation of Antibody Molecules
Roy Jefferis
Bioinformatics Tools for Analysis of Antibodies
Andrew C. R. Martin, James Allen
How to use IMGT for Therapeutic Antibody Engineering
Marie-Paul Lefranc
PART 2: MODIFIED ANTIBODIES
Bispecific Antibodies
Roland E. Kontermann, Dafne Müller
Single Domain Antibodies: An Overview
Carrie Enever, Edward Coulstock, Malgorzata Pupecka-Swider, B. Hamilton
Antibody-Drug Conjugates (ADCs): New Frontier in Cancer Therapeutics
Ravi Chari, Rajeeva Singh, John M. Lambert
Antibody Targeted Drugs: From Chemical Immunoconjugates to Recombinant Fusion Proteins
Jürgen Krauss, Athanasios Mavratzas, Michaela A. E. Arndt, Stefan Kiesgen
PART 3: EMERGING TECHNOLOGIES
Emerging Technologies for Antibody Selection
Mike Taussig, Mingyue He
Anti-idiotypic Antibodies
Alejandro Lopez-Requena, Oscar Roberto Burrone, Rolando Perez
Non-antibody Scaffolds as Alternative Therapeutic Agents
Arne Skerra, Markus Fiedler
Antibody Directed Enzyme Prodrug Therapy (ADEPT)
Surinder K. Sharma, Kerry A. Chester, Kenneth D. Bagshawe
Engineered Antibody Domains as Candidate Therapeutics
Weizao Chen, Ponraj Prabakaran, Dimiter S. Dimitrov
Chimeric Antigen Receptors - "CARs"
Thomas Schirrmann, Ulf Petrausch
Emerging Alternative Production Systems
Thomas Jostock, Benjamin Sommer, Holger Laux, Andre Frenzel
VOLUME II: CLINICAL DEVELOPMENT OF ANTIBODIES
PART 4: THE WAY INTO THE CLINIC
Process Development and Manufacturing of Therapeutic Antibodies
Hitto Kaufmann, Alexander Jacobi, Barbara Enenkel, Patrick Garidel, Christian Eckermann, Mathias Knappenberger, Ingo Presser
The Immunogenicity of Therapeutic Antibodies
Melody Sauerborn
Biosimilar Monoclonal Antibodies
Susanne D. Pippig, Carsten Brockmeyer, Robert E. Zoubek
Patent Issues Relating to Therapeutic Antibodies
Michael Braunagel, Barbara Rigby, Deborah Owen
PART 5: THERAPEUTIC ANTIBODY PIPELINE
Monoclonal Antibodies in Phase III Clinical Trials
Peter Markus Deckert, Ulf Petrausch
Antibodies in Early Phase Clinical Studies: Cancer Therapy
Anthony Olszanski, Matthew Zibelman, Hossein Borghaei
Targeting Angiogenesis by Therapeutic Antibodies
Thomas Effert, Onat Kadioglu, Ean Jeong Seo
Antibodies in Phase 3: Immunological Disorders
Penelope Ward, Mark Bodmer
Monoclonal Antibodies in Phase 1 and 2 Studies for Immunological Disorders
Frank Brennan
MAbs Targeting Soluble Mediators in Phase 1 and 2 Clinical Studies Immunological Disorders
Frank Brennan
T Cell Inhibitors in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
B Cell Inhibitors in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
Inhibitors of Leukocyte Adhesion and Migration in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
Toll-Like Receptor Inhibitors in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
IgE Inhibitors in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
Complement Inhibitors in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
mAbs Targeting Apoptosis, Angiogenesis Inhibitors and other mAbs in Phase 1 and 2 Clinical Studies for Immunological Disorders
Frank Brennan
Antibodies in Clinical Study: Infectious Diseases
Guillaume Descoubeaux
Immunotherapeutics for Neurological Disorders
Anne Messer, Kevin Manley, Cynthia A. Lemere
PART 6: GAINING MARKETING APPROVAL
Regulatory Considerations in the Development of Monoclonal Antibodies for Diagnosis and Therapy
Marjorie A. Shapiro, Patrick G. Swann, Stacey Ricci
Regulatory Review: Clinical to Market Transition
Gabriele Dallmann
Monoclonal antibody nomenclature for clinical studies (USA)
Stephanie Shubat
VOLUME III: APPROVED THERAPEUTIC ANTIBODIES
PART 7: APPROVED THERAPEUTIC ANTIBODIES
Oligoclonal and Polyclonal Antibody Preparations
Mark C. Glassy, Rishab K. Gupta
Adalimumab (Humira)
Janice Reichert
Alemtuzumab (Lemtrada, MabCampath)
Janice Reichert, Thomas Elter, Michael Hallek
Basiliximab and Daclizumab
Nasimul Ahsan, Burcin Taner, Nadim Mahmud
Belimumab (Benlysta)
David P. D´Cruz, Pamela M.K. Lutalo, Natasha Jordan, Thi-Sau Migone
Brentuximab vedotin (Adcetris)
Neils van de Donk
Canakinumab (ILARIS)
Hermann Gram
Catumaxomab (Removab) - Trifunctional Antibodies: Combining Direct Tumor Cell Killing with Therapeutic Vaccination
Horst Lindhofer, M. Stanglmaier, R. Buhmann, M. Jäger, D. Klunker, P. Ruf, J. Hess
Cetuximab (Erbitux)
Sonja Wilke, Michael Hust
Denosumab (Prolia)
Torsten Meyer
Efalizumab (Raptiva)
Karlheinz Schmitt-Rau
Calicheamycin Conjugates: Gemtuzumab Ozogamicin (Mylotarg), Inotuzumab Ozogamicin
Martin Gramatzki, Matthias Peipp
Golimumab (Simponi)
Janice Reichert, Sohini Mazumdar
Yttrium-90 Ibritumomab Tiuxetan (Zevalin)
Karin Hohloch, Björn Chapuy, Lorenz Trümper
Infliximab (Remicade)
Christian Antoni
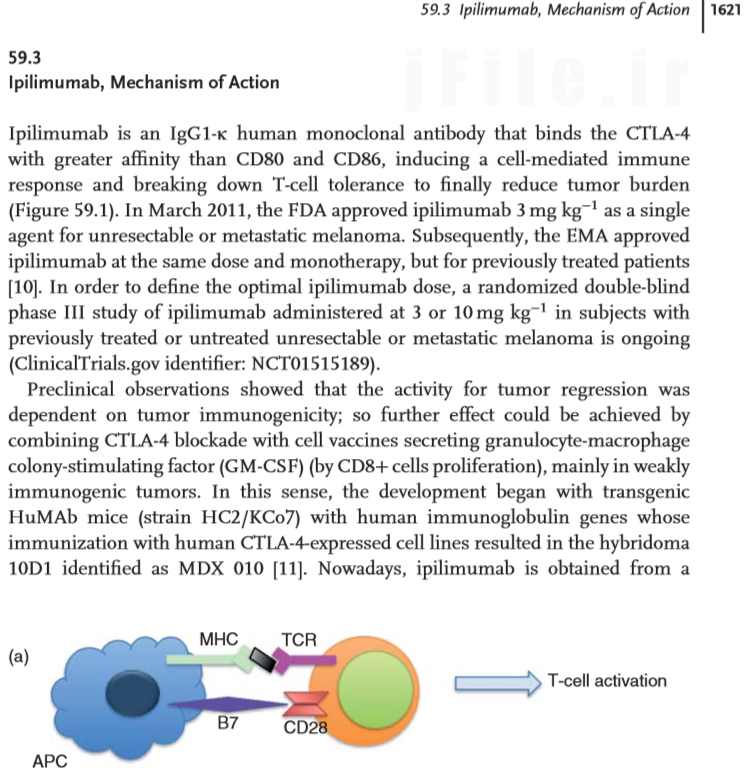
Ipilimumab (Yervoy)
Javier Puente, Teresa Alonso Gordoa, Eduardo Diaz-Rubio
Muromonab-CD3 (Orthoclone OKT3)
Janice Reichert, Harald Becker
Nimotuzumab: A Humanized Anti-EGFR Antibody
Tania Crombet
Obinutuzumab
Christian Klein, Marina Bacac, Pablo Umana, Michael Wenger
VOLUME IV: APPROVED THERAPEUTIC ANTIBODIES AND IN VIVO DIAGNOSTICS
Ofatumumab: A Next-Generation Human Therapeutic CD20 Antibody with Potent Complement-Dependent Cytotoxicity
Ronald P. Taylor, Margaret A. Lindorfer, Joost Bakker, Paul Parren
Omalizumab (Xolair) - Anti-Immunoglobulin E Treatment in Allergic Diseases
Klaus Kroegel, Martin Foerster
Palivizumab
Louis Bont
Panitumumab (Vectibix) - A Treatment for Metastatic Colorectal Cancer
Jonas Kügler
Pertuzumab (Perjeta)
Jose Angel Garcia-Saenz, Fernando Moreno Anton, Coralia Bueno Muino
Ranibizumab (Lucentis): A New Anti-angiogenic Treatment in Ophthalmology
Nicolas Leveziel, Marc Ohresser, Gilles Paintaud
Raxibacumab - Human Monoclonal Antibody Against Anthrax Toxin
Sally Bolmer, Thi-Sau Migone
Rituximab (Rituxan)
Stefan Dübel, Axel Boehnke, Michael Wenger
Tocilizumab (Actemra)
Graeme Jones
Trastuzumab (Herceptin) and Ado-Trastuzumab Emtansine (T-DM1)
M. H. Ruhe Chowdhury, Paul Ellis
Ustekinumab (Stelara)
Stefan Dübel, Oya Cingoz, Janice Reichert
Abciximab, Bevacizumab, Certolizumab pegol, Eculizumab, Natalizumab
Janice Reichert
Itolizumab (Alzumab), Mogamulizumab (Poteligeo) and Tositumomab (Bexxar)
Stefan Dübel
PART 8: IN VIVO DIAGNOSTICS
Radiolabeled Antibodies for Diagnostic Imaging
Christopher J. Palestro
Appendix
Index
companies.
Author Information
Stefan Dübel received his Ph.D. at the Center for Molecular Biology in Heidelberg, Germany, before moving on to the German Cancer Research Center in 1989 where he started research on the development of antibody libraries with naive or synthetic CDRs. In the following years, he substantially participated in the invention of antibody phage display, as well as other recombinant antibody technologies and the development of various antibody fusion proteins. In 1996, he moved to the University of Heidelberg to establish the antibody engineering group at the Institute of Molecular Genetics. He was appointed full professor at the Technische Universität Braunschweig in 2002, where he currently is head of the Department of Biotechnology and managing director of the Institute of Biochemistry, Biotechnology and Bioinformatics. He has co-authored more than 100 original papers, many patents, as well as several acknowledged textbooks in the field of recombinant antibodies. He is also on the editorial board of several scientific journals and cofounder of two antibody engineering companies.
Janice M. Reichert is an internationally recognized expert in the development of antibody therapeutics. She is Founder and Managing Director of Reichert Biotechnology Consulting LLC, a pharmaceutical business intelligence research firm, and the founding Editor-in-Chief of "mAbs", a peer-reviewed, PubMed-indexed biomedical journal that focuses on topics relevant to antibody research and development. Dr. Reichert has published extensively on development trends for antibody therapeutics and she has presented her research results as an invited speaker at conferences held worldwide. She is President of The Antibody Society and serves on the editorial boards of several biomedical journals. Dr. Reichert received her Ph.D. from the University of Pennsylvania and her post-doctoral training at Harvard Medical School.
نمونه کیفیت صفحات کتاب